Recent advancements have broadened the spectrum of treatments for advanced NETs, encompassing a range of therapies that include peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE. The approval of 177Lu-DOTATATE after the NETTER-1 clinical trial marked a significant milestone, offering new hope for patients with advanced NETs. By improving progression-free survival (PFS), this therapy has become a critical component of the treatment regimen for many somatostatin receptor-positive NETs.
However, for many advanced NETs, disease progression is an ongoing challenge that requires further exploration of treatment options, including the possibility of PRRT retreatment. A recent study from the Excel Diagnostics and Nuclear Oncology Center in Houston, Texas, sheds light on the real-world effectiveness and safety of 177Lu-DOTATATE retreatment for progressive NETs in the United States.
This outcomes study retrospectively examined the outcomes of 31 patients who underwent retreatment with 177Lu-DOTATATE at a single medical center in Houston. The majority of these patients had previously received multiple forms of treatment, and all had metastases to the bone, liver, or other sites. The most common finding after the initial PRRT treatment and retreatment was stable disease. The median PFS was 20.2 months after the initial treatment and 9.6 months after the retreatment.
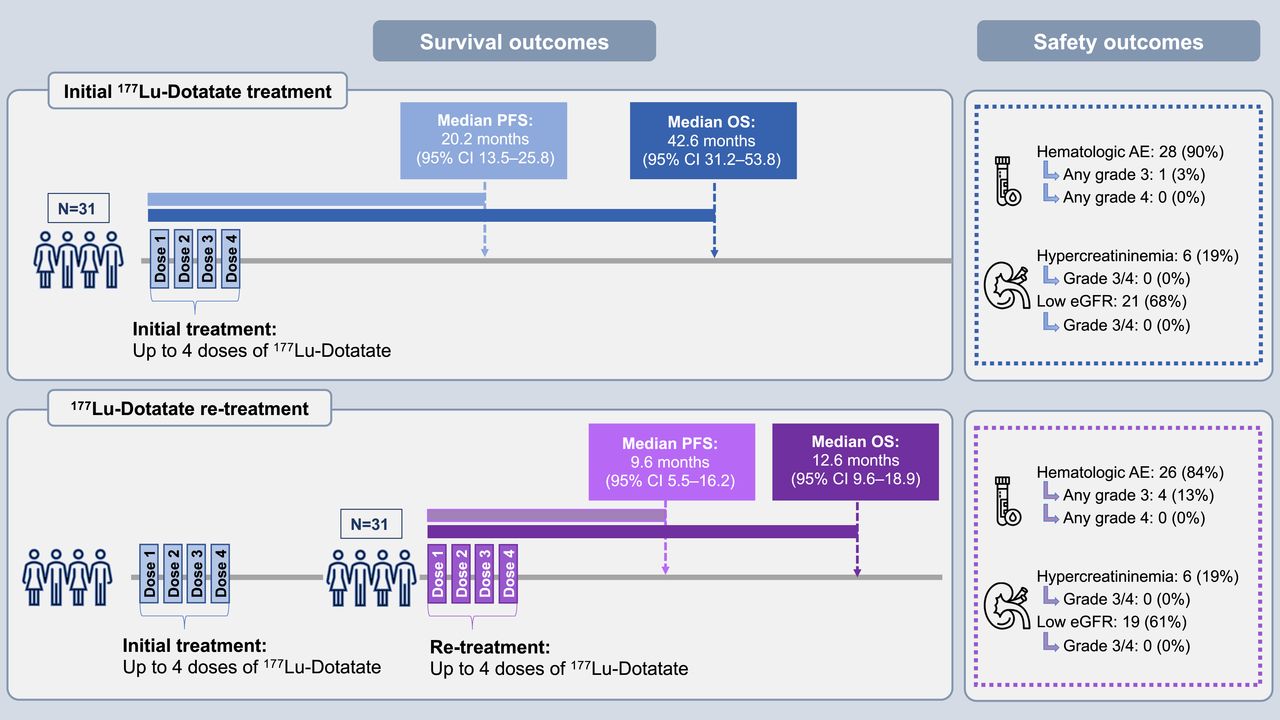
Figure 1. This research was originally published in JNM. Ebrahim S. Delpassand, Soheil M. Yazdi, Shashank Ghantoji, Antonio Nakasato, Corinne Strickland, Rodolfo Nunez, Afshin Shafie, Susan Cork, Clare Byrne, Jackson Tang, Jeetvan Patel. Effectiveness and Safety of Retreatment with 177Lu-DOTATATE in Patients with Progressive Neuroendocrine Tumors: A Retrospective Real-World Study in the United States. J Nucl Med. 2024; 00:1-7. DOI: 10.2967/jnumed.123.265703 © SNMMI.
The findings indicate that despite the advanced stage of disease at the time of retreatment, patients could still achieve disease control with retreatment. Further, the safety profile of retreatment was encouraging, showing only minimal severe adverse events.
This study does have limitations, including a small sample size and retrospective design, which could introduce potential biases. The follow-up duration may also have been too short to capture all adverse events. Despite these challenges, this study represents one of the largest to date to analyze PRRT retreatment outcomes in the U.S. PRRT retreatment is rare because it is not universally eligible for reimbursement by insurance.
More research is needed to determine whether PRRT retreatment is a valuable option for patients who have exhausted other therapies. A prospective, randomized phase II trial called NET RETREAT is now ongoing to assess 177Lu-DOTATATE retreatment versus everolimus, which should provide the information clinicians will need to make more informed treatment decisions.
As we continue to navigate the complexities of NET management, the Neuroendocrine Tumor Research Foundation remains committed to supporting research and education that will lead to better outcomes for NET patients. The findings from this study not only contribute to our growing knowledge base but also highlight the importance of ongoing research and innovation in the quest to improve the lives of those affected by NETs.
Read the full research article here.
