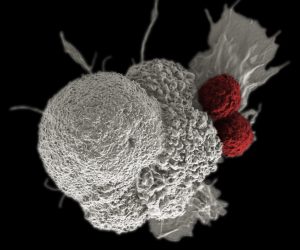
Imagine being able to program your immune system to launch one million minuscule heat-seeking missiles, whose sole purpose is to find and kill cancer cells. This is the basic premise behind an emerging form of immunotherapy, called CAR T-cell therapy, which genetically modifies an individual’s immune system to find, bind to, and kill cancer cells.
The evolution of CAR T-Cell Therapy
A recent FDA approval of CAR T-cell therapy in childhood leukemia has put this breakthrough approach in the spotlight. NETRF has funded multiple research studies on CAR T-cell therapy in NETs since 2014. The Foundation’s first grant was to a pioneer of this therapy Carl June, M.D., and his colleagues Xianxin Hua, M.D., Ph.D., and David Metz, M.D., at the University of Pennsylvania. These colleagues continue to collaborate on NET research, with Dr. Hua leading an ongoing study.
“These recent advances raise an exciting possibility that NETs can be specifically targeted via specific CAR T cells to effectively eradicate NET cells,” said Xianxin Hua, M.D., Ph.D.
Most of the early CAR T-cell research focused on blood cancers, especially lymphoiblast leukemia, because these leukemia cells express a highly lineage-specific cell surface antigen that can be targeted by CAR T cells to eradicate the antigen-bearing cells including the leukemia cells. While NET cancer cells do not express a NET cell-specific cell surface antigen, NET cells tend to express abnormally high levels of somatostatin receptors (SSTRs) on their surface. Researchers hope that SSTR can serve as the antigen that attracts the killer CAR T cells.
What is CAR T-Cell Therapy?

As its name implies, the backbone of CAR T-cell therapy is genetically engineered T cells, which work as the workhorses of the immune system to kill cells with surface antigens that can be specifically recognized by the engineered CAR T cells. The therapy requires drawing blood from patients and separating out the T cells. Next, the T cells are genetically engineered to produce receptors on their surface called chimeric antigen receptor, or CARs, which is tethered to T cell-activating modules. The CAR-expressing T cells, or CAR T cells, are programmed to cling to the specific antigen found on the surface of the cancer cell. Then they are “expanded” in the laboratory to created hundreds of millions of cancer-killing cells. The final step is the infusion of the CAR T cells into the patient. If all goes as planned, the engineered cells further multiply in the patient’s body and, with guidance from their engineered receptor, recognize and kill cancer cells that harbor the SSTR antigen on their surfaces.
CAR T-Cell Therapy in NETs
The first step in using this technology in NETs is to develop receptors to recognize SSTR as an antigen on surface of the neuroendocrine tumor cell. Dr. Hua and colleagues have developed a CAR system that can attach to SSTR-expressing tumor cells and kill the cancer cell. They have successfully tested the receptors in cultures, laboratory models, and are preparing next to test them in freshly isolated human tumor cells.
“These studies will likely lead to the development of an entirely new and more effective therapy for NET patients who have failed previous treatments and save their life,” said Dr. Hua.
Learn more about NETRF-funded research. Support research to cure NETs.
