
Unraveling the Causes of Small Intestinal NETs
The first step in curing a disease is understanding it. Unfortunately, there is a lot we still don’t know about small-intestinal NETs (SI-NETs). That’s why

The first step in curing a disease is understanding it. Unfortunately, there is a lot we still don’t know about small-intestinal NETs (SI-NETs). That’s why

NETRF awarded six new research grants totaling $1.85 million to leading academic institutions around the world. The goal of the funding is to improve current treatments for neuroendocrine tumors (NETs), an uncommon and poorly understood cancer, which occurs in the body’s hormone-producing cells.
New treatment for GI and pancreatic NETs The U.S. Food and Drug Administration (FDA) has approved Peptide Receptor Radionuclide Therapy (PRRT) with a new radiopharmaceutical for

World renown laboratory in the Netherlands to grown min-organs in Petri dishes to speed up NET drug testing.

Even those who did not know her were inspired by the stories of Meg’s positive, generous spirit, her sense of humor, and ability to squeeze so much life into her 31+years. Nurse Meg took care of us all and is forever in our hearts—forever running alongside encouraging us to dream bigger and live stronger—and find and inspire laughter and joy each day.

Much about a carcinoid cancer cell remains a mystery. For that reason, NETRF funds research to understand how and why a neuroendocrine cancer cell comes

NETRF in collaboration with NANETS awarded the 2017 Basic Translational Science Investigator grant to Brian R. Untch, M.D., at Memorial Sloan Kettering Cancer Center for his proposal, “Enhancing Peptide Receptor Radionuclide Therapy in Well-Differentiated Pancreatic Neuroendocrine Tumors.”

There is a critical need to develop improved diagnostic tools for non-invasive, early detection of NETs in a broader range of patients. New grant-funded research will work towards this goal.
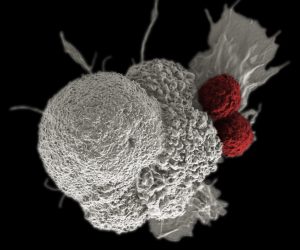
Imagine being able to program your immune system to launch one million minuscule heat-seeking missiles, whose sole purpose is to find and kill cancer cells. This is the basic premise behind an emerging form of immunotherapy, called CAR T-cell therapy, which genetically modifies an individual’s immune system to find, bind to, and kill cancer cells.